BEST-J score
Published by:
Yoichi Kakuta

Description
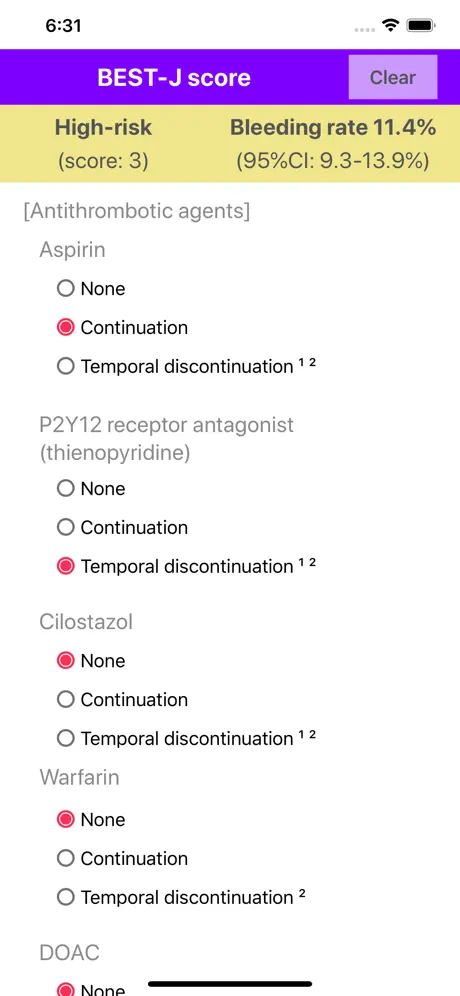
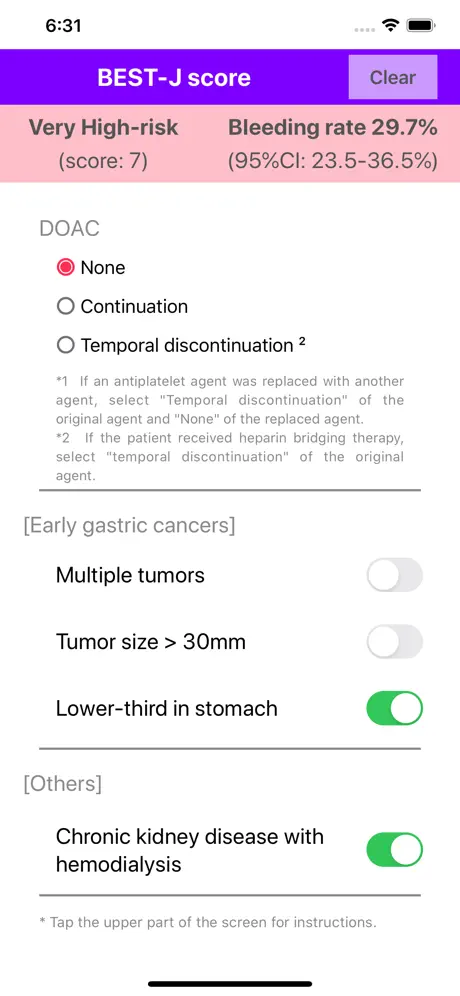
Bleeding after ESD occurs in 4.1%–8.5% of patients who undergo gastric ESD. The "BEST-J score" is a prediction model to stratify the risk of bleeding after ESD for early gastric cancers. Nine factors were weighted with point values: 4 points for anticoagulants (warfarin, direct oral anticoagulants), 3 points for chronic kidney disease with hemodialysis, 2 points each for P2Y12 receptor antagonist (thienopyridine) and aspirin, 1 point each for cilostazol, a tumor size >30 mm, lower-third in tumor location, and presence of multiple tumors, and −1 point for interruption of each kind of antithrombotic agents. The patients were categorized into four bleeding risk groups: low (0–1 points: 2.8% risk), intermediate (2 points: 6.1%), high (3–4 points: 11.4%) and very high (≥5 points: 29.7%).
*1. If an antiplatelet agent was replaced with another agent, select "Temporal discontinuation" of the original agent and "None" of the replaced agent. For example, if P2Y12 receptor antagonist is replaced with aspirin, select "Temporal discontinuation" of P2Y12 receptor antagonist and "None" of aspirin.
*2. If the patient received heparin bridging therapy, select "Temporal discontinuation" of the original agent. For example, if warfarin is bridged with heparin, select "Temporal discontinuation" of warfarin.
*3. Time of discontinuing antithrombotic agents before ESD is based on the Japanese guidelines (Dig Endosc. 2014; 26: 1-14.): aspirin, 3−5 days; P2Y12 receptor antagonist (thienopyridine), 5−7 days; cilostazol, 1 day; warfarin, 3−5 days; DOAC, 1−2 days.
For more information, see the reference below.
Gut. 2020 [Epub ahead of print]
A prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score.
Hatta W, Tsuji Y, Yoshio T, Kakushima N, Hoteya S, Doyama H, Nagami Y, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Koike T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Sugimoto M, Yamaguchi S, Michida T, Yada T, Asahina Y, Narasaka T, Kuribayashi S, Kiyotoki S, Mabe K, Nakamura T, Nakaya N, Fujishiro M, Masamune A.
You can view the application help by tapping a title bar.
DISCLAIMER: This application is intended for educational or research purposes only. It does not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment.
[Developer]
2020 東北大学病院消化器内科・大腸グループ
2020 Kenichi Negoro, MD, PhD
2020 Yoichi Kakuta, MD, PhD
2020 Waku Hatta, MD, PhD (Consultant)
2020 Xiaoyi Jin, MD, PhD (Translation)
http://www.gastroente.med.tohoku.ac.jp/
[email protected]
Hide
Show More...
*1. If an antiplatelet agent was replaced with another agent, select "Temporal discontinuation" of the original agent and "None" of the replaced agent. For example, if P2Y12 receptor antagonist is replaced with aspirin, select "Temporal discontinuation" of P2Y12 receptor antagonist and "None" of aspirin.
*2. If the patient received heparin bridging therapy, select "Temporal discontinuation" of the original agent. For example, if warfarin is bridged with heparin, select "Temporal discontinuation" of warfarin.
*3. Time of discontinuing antithrombotic agents before ESD is based on the Japanese guidelines (Dig Endosc. 2014; 26: 1-14.): aspirin, 3−5 days; P2Y12 receptor antagonist (thienopyridine), 5−7 days; cilostazol, 1 day; warfarin, 3−5 days; DOAC, 1−2 days.
For more information, see the reference below.
Gut. 2020 [Epub ahead of print]
A prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score.
Hatta W, Tsuji Y, Yoshio T, Kakushima N, Hoteya S, Doyama H, Nagami Y, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Koike T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Sugimoto M, Yamaguchi S, Michida T, Yada T, Asahina Y, Narasaka T, Kuribayashi S, Kiyotoki S, Mabe K, Nakamura T, Nakaya N, Fujishiro M, Masamune A.
You can view the application help by tapping a title bar.
DISCLAIMER: This application is intended for educational or research purposes only. It does not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment.
[Developer]
2020 東北大学病院消化器内科・大腸グループ
2020 Kenichi Negoro, MD, PhD
2020 Yoichi Kakuta, MD, PhD
2020 Waku Hatta, MD, PhD (Consultant)
2020 Xiaoyi Jin, MD, PhD (Translation)
http://www.gastroente.med.tohoku.ac.jp/
[email protected]
Screenshots
BEST FAQ
-
Is BEST free?
Yes, BEST is completely free and it doesn't have any in-app purchases or subscriptions.
-
Is BEST legit?
Not enough reviews to make a reliable assessment. The app needs more user feedback.
Thanks for the vote -
How much does BEST cost?
BEST is free.
-
What is BEST revenue?
To get estimated revenue of BEST app and other AppStore insights you can sign up to AppTail Mobile Analytics Platform.

User Rating
App is not rated in Kuwait yet.

Ratings History
BEST Reviews
Store Rankings

Ranking History
App Ranking History not available yet

Category Rankings
App is not ranked yet
BEST Installs
Last 30 daysBEST Revenue
Last 30 daysBEST Revenue and Downloads
Gain valuable insights into BEST performance with our analytics.
Sign up now to access downloads, revenue, and more.
Sign up now to access downloads, revenue, and more.
App Info
- Category
- Health Fitness
- Publisher
-
Yoichi Kakuta
- Languages
- English, Japanese, Chinese
- Recent release
- 1.0 (5 years ago )
- Released on
- Jun 4, 2020 (5 years ago )
- Also available in
- Japan, China, Ecuador, Egypt, Malaysia, Romania, United States, Taiwan, Peru, Spain, Greece, New Zealand, South Korea, Vietnam, Indonesia, Kuwait, Kazakhstan, Australia, Argentina
- Last Updated
- 2 weeks ago
This page includes copyrighted content from third parties, shared solely for commentary and research in accordance with fair use under applicable copyright laws. All trademarks, including product, service, and company names or logos, remain the property of their respective owners. Their use here falls under nominative fair use as outlined by trademark laws and does not suggest any affiliation with or endorsement by the trademark holders.