Chemical Kinetics
Published by: Roman Volinsky

Description
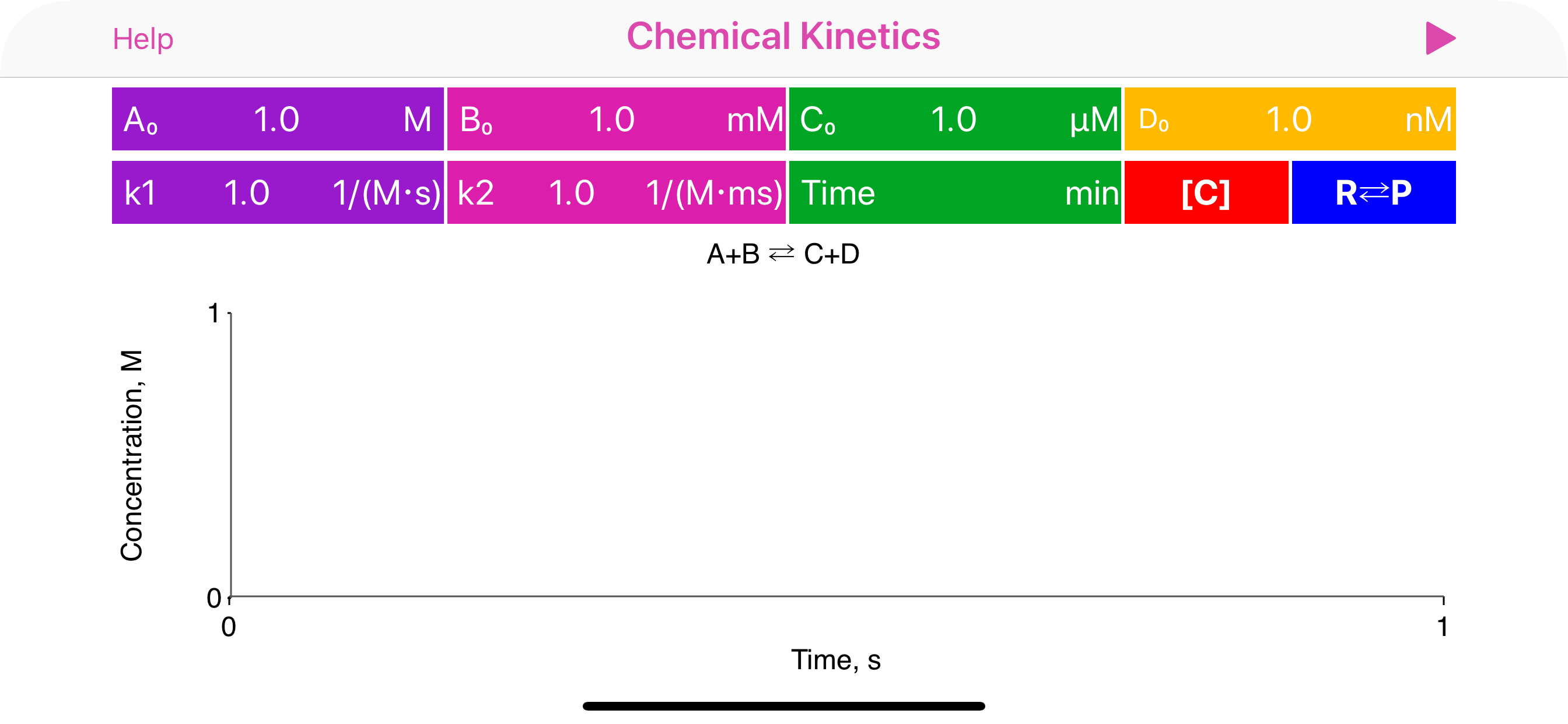
The “Chemical Kinetics” is an ultimate tool for solving all kind of chemical kinetics problems. It deals with time dependent quantitative analysis of chemical reactions. Specifically, this application solves chemical reaction rate equations and provides graphical representation of reagent and product concentrations vs time. Additionally, app calculates half-life times and equilibrium constants, where it is appropriate.
Theoretical Background:
For a general chemical reaction:
aA + bB => cC + dD,
the reaction rate is defined by:
rate=k * A^x * B^y
where k – is a rate coefficient and sum of powers x and y determines the reaction order. Importantly, actual powers x and y may differ from reaction coefficients a and b, due to overall reaction mechanism complexity.
The application features:
· User is provided with list of basic mono-step unidirectional and equilibrium reactions, where reaction coefficients are actually represent powers and therefore define reaction order:
2A => B, rate = k[A]^2
A+B = C+D, rate = k1[A] [B] - k2[C] [D]
· k1 is a rate coefficient of forward reaction in equilibrium process or rate coefficient for first step in multistep process. Similarly, k2, when available, is a rate coefficient of backward reaction in equilibrium process or rate coefficient for second step in multistep process.
· To start plotting the reaction data initially or after updating the concentration or coefficient fields, user is requested to tap Run button. Run button is greyed out immediately after successful calculation.
· Complete reaction information is provided on top of the plot: concentrations at certain time point (defined by user or provided automatically), optionally equilibrium constant value and half-life time.
· When time field is empty, the app automatically determines the time range. To redefine reaction time limit and find out component concentrations at the altrnative time point, user may update the time field.
· Significant attention should be paid to units of rate coefficients. App automatically changes the set of available units as per order of reaction.
Units acronyms: d - days, h - hours, a - atmosphere, s - seconds, min - minutes, y - years, M -molars, Pa - pascals.
· The basic conversions are as follows:
M-1·s-1 = (1e-3) mM-1·s-1 = (60e-3) mM-1·min-1 = (3600) M-1·h-1
1 atm=101325 Pa
Hide
Show More...
Theoretical Background:
For a general chemical reaction:
aA + bB => cC + dD,
the reaction rate is defined by:
rate=k * A^x * B^y
where k – is a rate coefficient and sum of powers x and y determines the reaction order. Importantly, actual powers x and y may differ from reaction coefficients a and b, due to overall reaction mechanism complexity.
The application features:
· User is provided with list of basic mono-step unidirectional and equilibrium reactions, where reaction coefficients are actually represent powers and therefore define reaction order:
2A => B, rate = k[A]^2
A+B = C+D, rate = k1[A] [B] - k2[C] [D]
· k1 is a rate coefficient of forward reaction in equilibrium process or rate coefficient for first step in multistep process. Similarly, k2, when available, is a rate coefficient of backward reaction in equilibrium process or rate coefficient for second step in multistep process.
· To start plotting the reaction data initially or after updating the concentration or coefficient fields, user is requested to tap Run button. Run button is greyed out immediately after successful calculation.
· Complete reaction information is provided on top of the plot: concentrations at certain time point (defined by user or provided automatically), optionally equilibrium constant value and half-life time.
· When time field is empty, the app automatically determines the time range. To redefine reaction time limit and find out component concentrations at the altrnative time point, user may update the time field.
· Significant attention should be paid to units of rate coefficients. App automatically changes the set of available units as per order of reaction.
Units acronyms: d - days, h - hours, a - atmosphere, s - seconds, min - minutes, y - years, M -molars, Pa - pascals.
· The basic conversions are as follows:
M-1·s-1 = (1e-3) mM-1·s-1 = (60e-3) mM-1·min-1 = (3600) M-1·h-1
1 atm=101325 Pa
Screenshots
Chemical Kinetics FAQ
-
Is Chemical Kinetics free?
Yes, Chemical Kinetics is completely free and it doesn't have any in-app purchases or subscriptions.
-
Is Chemical Kinetics legit?
Not enough reviews to make a reliable assessment. The app needs more user feedback.
Thanks for the vote -
How much does Chemical Kinetics cost?
Chemical Kinetics is free.
-
What is Chemical Kinetics revenue?
To get estimated revenue of Chemical Kinetics app and other AppStore insights you can sign up to AppTail Mobile Analytics Platform.

User Rating
App is not rated in Saudi Arabia yet.

Ratings History
Chemical Kinetics Reviews
No Reviews in Saudi Arabia
App doesn't have any reviews in Saudi Arabia yet.
Store Rankings

Ranking History
App Ranking History not available yet

Category Rankings
App is not ranked yet
Chemical Kinetics Competitors
| Name | Downloads (30d) | Monthly Revenue | Reviews | Ratings | Recent release | |
|---|---|---|---|---|---|---|
|
Read Chemistry Mcqs!
Chemistry Mcqs Prep 2021
|
Unlock
|
Unlock
|
0
|
|
3 years ago | |
|
Rate of Chemical Reaction
|
Unlock
|
Unlock
|
0
|
|
2 years ago | |
|
Handbook Of Chemistry
Chemistry Handbook
|
Unlock
|
Unlock
|
0
|
|
3 years ago | |
|
Chemistry AR+
View Atoms, Molecules in AR
|
Unlock
|
Unlock
|
0
|
|
3 years ago | |
|
Redox Reaction - Chemistry
|
Unlock
|
Unlock
|
0
|
|
2 years ago | |
|
Core-apps Events
|
Unlock
|
Unlock
|
0
|
|
1 month ago | |
|
CloudLabs Chemical Reactions
N/A
|
Unlock
|
Unlock
|
0
|
|
6 months ago | |
|
CloudLabs Ions Equilibrium
|
Unlock
|
Unlock
|
0
|
|
6 months ago | |
|
AP Chemistry Guided Sims
Rates, Kinetics, Equilibrium
|
Unlock
|
Unlock
|
0
|
|
3 years ago | |
|
Chemistry Chapters
|
Unlock
|
Unlock
|
0
|
|
1 year ago |
Chemical Kinetics Installs
Last 30 daysChemical Kinetics Revenue
Last 30 daysChemical Kinetics Revenue and Downloads
Gain valuable insights into Chemical Kinetics performance with our analytics.
Sign up now to access downloads, revenue, and more.
Sign up now to access downloads, revenue, and more.
App Info
- Category
- Education
- Publisher
-
Roman Volinsky
- Languages
- English
- Recent release
- 3.7 (2 months ago )
- Released on
- Apr 13, 2015 (9 years ago )
- Also available in
- Indonesia, South Africa, United States, Thailand, Singapore, Saudi Arabia, New Zealand, Norway, Malaysia, Mexico, Kuwait, Japan, Italy, Ireland, United Arab Emirates, Greece, United Kingdom, France, Finland, Spain, Germany, Chile, Switzerland, Canada, Belgium, Australia, Austria
- Last Updated
- 1 month ago