LLS CAR T
Published by:
Leukemia & Lymphoma Society
Description
Chimeric Antigen Receptor T-Cell Therapy (CAR T-cell Therapy) is a treatment in which a patient’s own blood cells are genetically reprogrammed to find and kill cancer cells.
Using your smartphone, tablet, or other handheld device, download our interactive mobile app to learn about the process of CAR T-cell therapy. Our 3-D animation illustrates how T cells and CAR T cells travel through the bloodstream to seek out and eliminate blood cancer cells.
Hide
Show More...
Using your smartphone, tablet, or other handheld device, download our interactive mobile app to learn about the process of CAR T-cell therapy. Our 3-D animation illustrates how T cells and CAR T cells travel through the bloodstream to seek out and eliminate blood cancer cells.
Screenshots
LLS CAR T FAQ
-
Is LLS CAR T free?
Yes, LLS CAR T is completely free and it doesn't have any in-app purchases or subscriptions.
-
Is LLS CAR T legit?
Not enough reviews to make a reliable assessment. The app needs more user feedback.
Thanks for the vote -
How much does LLS CAR T cost?
LLS CAR T is free.
-
What is LLS CAR T revenue?
To get estimated revenue of LLS CAR T app and other AppStore insights you can sign up to AppTail Mobile Analytics Platform.

User Rating
App is not rated in Mexico yet.

Ratings History
LLS CAR T Reviews
Store Rankings

Ranking History
App Ranking History not available yet

Category Rankings
App is not ranked yet
LLS CAR T Installs
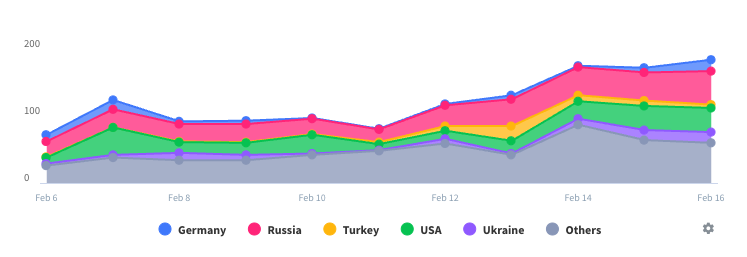
Last 30 daysLLS CAR T Revenue
Last 30 daysLLS CAR T Revenue and Downloads
Gain valuable insights into LLS CAR T performance with our analytics.
Sign up now to access downloads, revenue, and more.
Sign up now to access downloads, revenue, and more.
App Info
- Category
- Medical
- Publisher
- Leukemia & Lymphoma Society
- Languages
- English
- Recent release
- 1.0 (4 years ago )
- Released on
- Jul 6, 2020 (4 years ago )
- Also available in
- United States , United Arab Emirates , Brazil , Chile , United Kingdom , Mexico , New Zealand , South Africa
- Last Updated
- 3 months ago
This page includes copyrighted content from third parties, shared solely for commentary and research in accordance with fair use under applicable copyright laws. All trademarks, including product, service, and company names or logos, remain the property of their respective owners. Their use here falls under nominative fair use as outlined by trademark laws and does not suggest any affiliation with or endorsement by the trademark holders.