Description
Teckro transforms your clinical trial protocol into a hub of communication and collaboration. It’s a digital platform that connects all study stakeholders with the knowledge they need, when they need it. Drugs from trials powered by Teckro have been granted priority review and approval by regulators in the US and Europe. Thousands of investigators and 20,000+ sites around the world rely on Teckro to execute clinical trials from biopharma sponsors, including 12 out of the Top 20 pharma companies. We support all therapeutic areas, all phases, and any trial model, whether traditional, virtual, or a hybrid approach - if Teckro isn't used on your clinical trial, ask your study manager for it.
Hide
Show More...
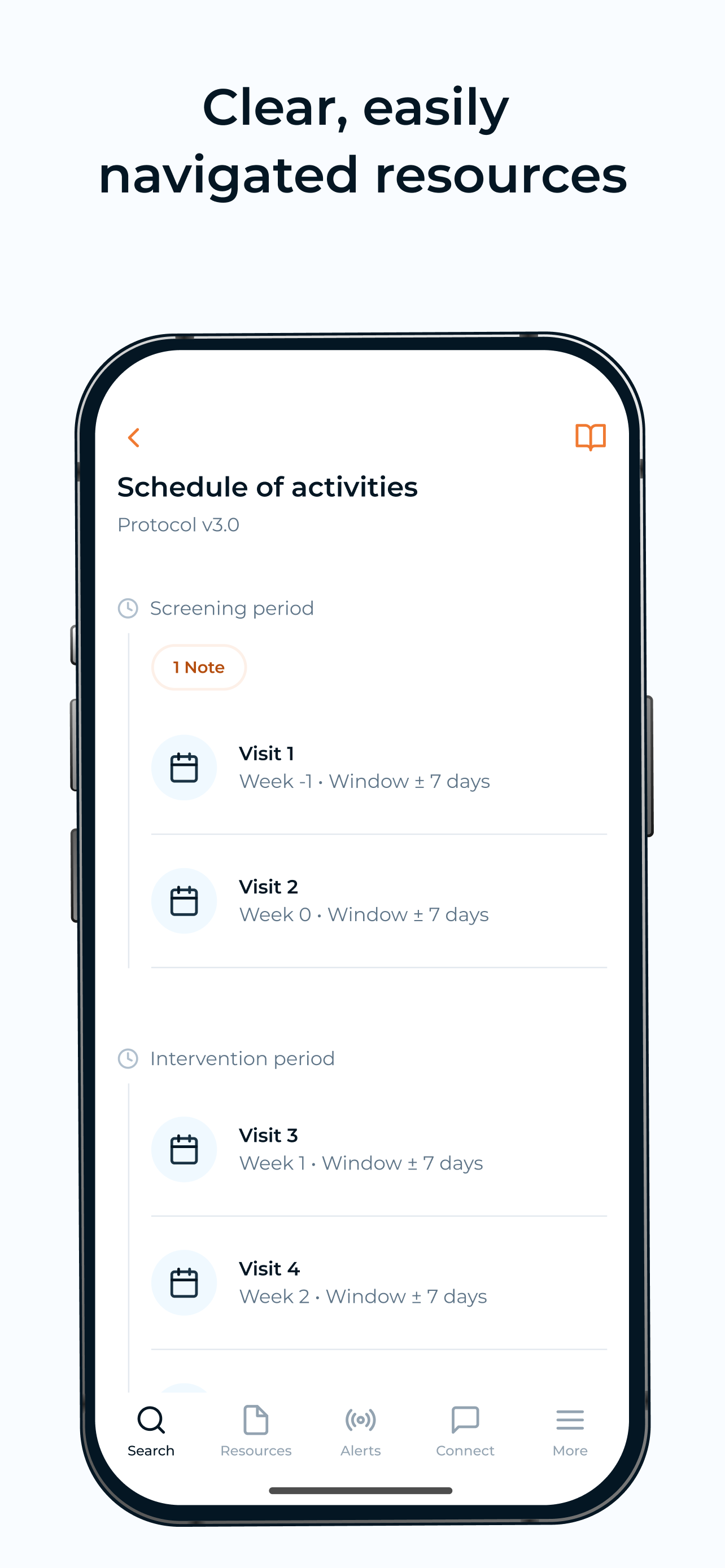
Screenshots
Teckro FAQ
-
Is Teckro free?
Yes, Teckro is completely free and it doesn't have any in-app purchases or subscriptions.
-
Is Teckro legit?
Not enough reviews to make a reliable assessment. The app needs more user feedback.
Thanks for the vote -
How much does Teckro cost?
Teckro is free.
-
What is Teckro revenue?
To get estimated revenue of Teckro app and other AppStore insights you can sign up to AppTail Mobile Analytics Platform.

User Rating
App is not rated in Romania yet.

Ratings History
Teckro Reviews
Store Rankings

Ranking History

Category Rankings
|
Chart
|
Category
|
Rank
|
|---|---|---|
|
Top Free
|

|
255
|
|
Top Free
|

|
303
|
|
Top Free
|

|
308
|
|
Top Free
|

|
323
|
|
Top Free
|

|
415
|
Teckro Competitors
| Name | Downloads (30d) | Monthly Revenue | Reviews | Ratings | Recent release | |
|---|---|---|---|---|---|---|
|
RapidAI
iSchemaView
|
Unlock
|
Unlock
|
0
|
|
1 week ago | |
|
ESMO Events App
|
Unlock
|
Unlock
|
0
|
|
1 month ago | |
|
Preclarus
Investigator Site Portal
|
Unlock
|
Unlock
|
0
|
|
1 month ago | |
|
CTCAE plus
Beyond paper-based management
|
Unlock
|
Unlock
|
0
|
|
1 month ago | |
|
SITC Immunotherapy Guidelines
|
Unlock
|
Unlock
|
0
|
|
8 months ago | |
|
ESMO Interactive Guidelines
|
Unlock
|
Unlock
|
0
|
1
|
1 year ago | |
|
ASCO Guidelines
|
Unlock
|
Unlock
|
0
|
|
11 months ago | |
|
Medidata Rave RCM
Regulated Content Management
|
Unlock
|
Unlock
|
0
|
|
2 years ago | |
|
Signant Health IRT
|
Unlock
|
Unlock
|
0
|
|
3 years ago | |
|
Veeva Snap
|
Unlock
|
Unlock
|
0
|
|
1 year ago |
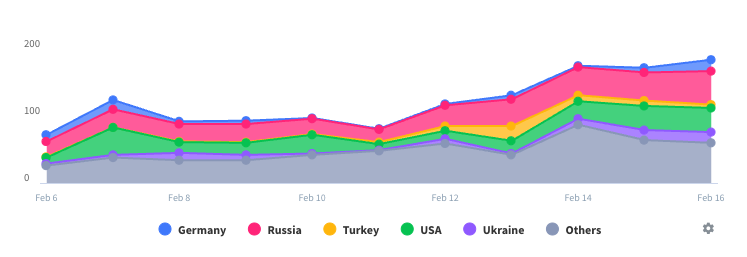
Teckro Installs
Last 30 daysTeckro Revenue
Last 30 daysTeckro Revenue and Downloads
Gain valuable insights into Teckro performance with our analytics.
Sign up now to access downloads, revenue, and more.
Sign up now to access downloads, revenue, and more.
App Info
- Category
- Medical
- Publisher
- Teckro
- Languages
- English
- Recent release
- 6.91.0 (1 week ago )
- Released on
- Apr 18, 2016 (8 years ago )
- Also available in
- Ireland, United States, Brazil, China, Argentina, Spain, Russia, Germany, Malaysia, Mexico, Italy, Canada, Australia, Lebanon, South Africa, Netherlands, Norway, New Zealand, Kazakhstan, Peru, Philippines, Pakistan, Poland, Portugal, Romania, Singapore, Thailand, Türkiye, Ukraine, Vietnam, United Arab Emirates, Egypt, Azerbaijan, Belgium, Switzerland, Chile, Colombia, Czechia, Denmark, Dominican Republic, Algeria, Ecuador, Kuwait, Finland, France, United Kingdom, Hong Kong SAR China, Hungary, Indonesia, Israel, India, Japan, South Korea
- Last Updated
- 1 day ago