Stoichiometry Pro
Veröffentlicht von:
Roman Volinsky

Downloads
Umsatz
Beschreibung
Stoichiometry Pro targets common stoichiometric tasks: balancing chemical reactions of unlimited number of components, finding molar masses, moles and masses of reactants and products and defining the limiting reagent, based on chemical formulas, stoichiometric coefficients, and any quantitative data provided.
The app analyses chemical reaction typed in “Reaction” field, balances it (if possible), finds molar masses and builds table with list of compounds (reagents and products coloured differently), for quantitative data input. All that triggered by pressing the = button.
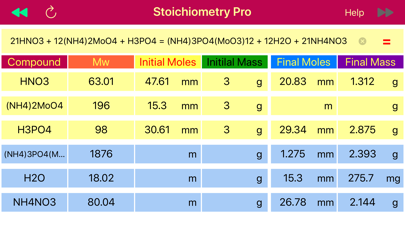
Example 1: How many moles of H3PO4 are needed in order to obtain 2.0 g of NH4NO3, in the reaction:
H3PO4 + (NH4)2MoO4 + HNO3 = (NH4)3PO4(MoO3)12 + NH4NO3 + H2O
Solution: The first step is balancing chemical reaction and finding molar masses, since moles-mass conversion is needed. Reaction is balanced and all molar masses are calculated automatically by pressing the “=” button.
H3PO4 + 12(NH4)2MoO4 + 21HNO3 = (NH4)3PO4(MoO3)12 +21NH4NO3 + 12H2O,
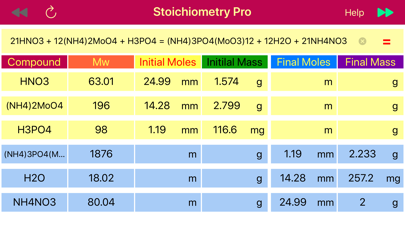
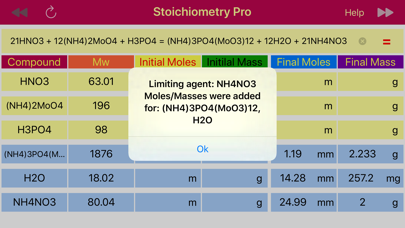
The next step is to find the moles of NH4NO3 from the mass (2.0 g), and then convert them to moles of H3PO4 based on 1:21 ratio obtained from the balanced reaction. Stoichiometry application provides data table that comprised of masses and mole rows for data available before reaction initiated and obtained or left after the reaction is accomplished. When calculate button that pointing towards products, ▶▶ is pressed, the final masses and moles are calculated from the initially provided amounts of reactant and products. On the opposite, when the calculate button pointing left,◀◀, is pressed, then initial reactant and product amounts that would be required to achieve the provided final state are calculated. The later is exactly matches our situation, as we need to find the initial amount of H3PO4 from the final, known NH4NO3 mass. So we set the NH4NO3 mass (2.0 g) to the final mass field and press calculate button ◀◀ to automatically fill the table with all masses and moles for all components. Apparently, 1.19 mmoles of H3PO4 are required to get 2.0 g of NH4NO3.
Important Points:
Either moles or masses can be filled in, but if both are provided and they don’t match, then the moles value will have a preference.
For balancing redox reactions please refer to Electrochemistry application.
Ausblenden
Mehr anzeigen...
The app analyses chemical reaction typed in “Reaction” field, balances it (if possible), finds molar masses and builds table with list of compounds (reagents and products coloured differently), for quantitative data input. All that triggered by pressing the = button.
Example 1: How many moles of H3PO4 are needed in order to obtain 2.0 g of NH4NO3, in the reaction:
H3PO4 + (NH4)2MoO4 + HNO3 = (NH4)3PO4(MoO3)12 + NH4NO3 + H2O
Solution: The first step is balancing chemical reaction and finding molar masses, since moles-mass conversion is needed. Reaction is balanced and all molar masses are calculated automatically by pressing the “=” button.
H3PO4 + 12(NH4)2MoO4 + 21HNO3 = (NH4)3PO4(MoO3)12 +21NH4NO3 + 12H2O,
The next step is to find the moles of NH4NO3 from the mass (2.0 g), and then convert them to moles of H3PO4 based on 1:21 ratio obtained from the balanced reaction. Stoichiometry application provides data table that comprised of masses and mole rows for data available before reaction initiated and obtained or left after the reaction is accomplished. When calculate button that pointing towards products, ▶▶ is pressed, the final masses and moles are calculated from the initially provided amounts of reactant and products. On the opposite, when the calculate button pointing left,◀◀, is pressed, then initial reactant and product amounts that would be required to achieve the provided final state are calculated. The later is exactly matches our situation, as we need to find the initial amount of H3PO4 from the final, known NH4NO3 mass. So we set the NH4NO3 mass (2.0 g) to the final mass field and press calculate button ◀◀ to automatically fill the table with all masses and moles for all components. Apparently, 1.19 mmoles of H3PO4 are required to get 2.0 g of NH4NO3.
Important Points:
Either moles or masses can be filled in, but if both are provided and they don’t match, then the moles value will have a preference.
For balancing redox reactions please refer to Electrochemistry application.
Screenshots
Stoichiometry Pro Häufige Fragen
-
Ist Stoichiometry Pro kostenlos?
Stoichiometry Pro ist nicht kostenlos (es kostet 6.98), enthält jedoch keine In-App-Käufe oder Abonnements.
-
Ist Stoichiometry Pro seriös?
Nicht genügend Bewertungen, um eine zuverlässige Einschätzung vorzunehmen. Die App benötigt mehr Nutzerfeedback.
Danke für die Stimme -
Wie viel kostet Stoichiometry Pro?
Der Preis von Stoichiometry Pro beträgt 6.98.
-
Wie hoch ist der Umsatz von Stoichiometry Pro?
Um geschätzte Einnahmen der Stoichiometry Pro-App und weitere AppStore-Einblicke zu erhalten, können Sie sich bei der AppTail Mobile Analytics Platform anmelden.

Benutzerbewertung
Die App ist in Singapur noch nicht bewertet.

Bewertungsverlauf
Stoichiometry Pro Bewertungen
Keine Bewertungen in Singapur
Die App hat noch keine Bewertungen in Singapur.
Store-Rankings

Ranking-Verlauf
App-Ranking-Verlauf noch nicht verfügbar

Kategorien-Rankings
App ist noch nicht gerankt
Stoichiometry Pro Konkurrenten
| Name | Downloads (30d) | Monatlicher Umsatz | Rezensionen | Bewertungen | Letzte Veröffentlichung | |
|---|---|---|---|---|---|---|
|
Balancing Chemical Equations
|
Freischalten
|
Freischalten
|
0
|
|
vor 6 Monaten | |
|
Chemistry - Stoichiometry
N/V
|
Freischalten
|
Freischalten
|
0
|
|
vor 3 Jahren | |
|
Chemical-Equation-Balance Pro
Stoichiometry Redox Net-Ionic
|
Freischalten
|
Freischalten
|
0
|
|
vor 5 Tagen | |
|
Atoms to Moles Calculator
Atoms to Moles Conversion
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
Molar Mass Calculator Pro
Make calculations easier
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
ODYSSEY Atomic Orbitals
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
ODYSSEY Polar Bonds- Molecules
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
ODYSSEY Electron Sharing
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
ODYSSEY Resonance
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren | |
|
ODYSSEY Functional Groups
|
Freischalten
|
Freischalten
|
0
|
|
vor 2 Jahren |
Stoichiometry Pro Installationen
Letzte 30 TageStoichiometry Pro Umsatz
Letzte 30 TageStoichiometry Pro Einnahmen und Downloads
Gewinnen Sie wertvolle Einblicke in die Leistung von Stoichiometry Pro mit unserer Analytik.
Melden Sie sich jetzt an, um Zugriff auf Downloads, Einnahmen und mehr zu erhalten.
Melden Sie sich jetzt an, um Zugriff auf Downloads, Einnahmen und mehr zu erhalten.
App-Informationen
- Kategorie
- Education
- Herausgeber
-
Roman Volinsky
- Sprachen
- English
- Letzte Veröffentlichung
- 2.1 (vor 3 Monaten )
- Veröffentlicht am
- Dec 31, 2020 (vor 3 Jahren )
- Auch verfügbar in
- Italien , Südafrika , Vereinigte Staaten , Thailand , Singapur , Schweden , Saudi-Arabien , Portugal , Neuseeland , Norwegen , Niederlande , Mexiko , Kuwait , Südkorea , Japan , Vereinigte Arabische Emirate , Irland , Griechenland , Vereinigtes Königreich , Frankreich , Finnland , Spanien , Dänemark , Deutschland , Schweiz , Kanada , Belgien , Australien , Österreich
- Zuletzt aktualisiert
- vor 1 Monat
This page includes copyrighted content from third parties, shared solely for commentary and research in accordance with fair use under applicable copyright laws. All trademarks, including product, service, and company names or logos, remain the property of their respective owners. Their use here falls under nominative fair use as outlined by trademark laws and does not suggest any affiliation with or endorsement by the trademark holders.
- © 2024 AppTail.
- Unterstützung
- Privacy
- Terms
- All Apps