Beschreibung
Thermochemistry helps in evaluation of enthalpy or heat release/absorption of a system undergoing numerous temperature changes and phase transitions. The calculations take advantage of known values of heat capacity at constant pressure (Cp) and molar or per gram enthalpy of phase transition. Amount of compound can be defined in grams or moles, in a way that Cp and enthalpy units would match.
App provides enthalpy values for each step. Negative enthalpy points to exothermic process – heat release, while positive one to endothermic - heat absorption. °C and K are interchangeable.
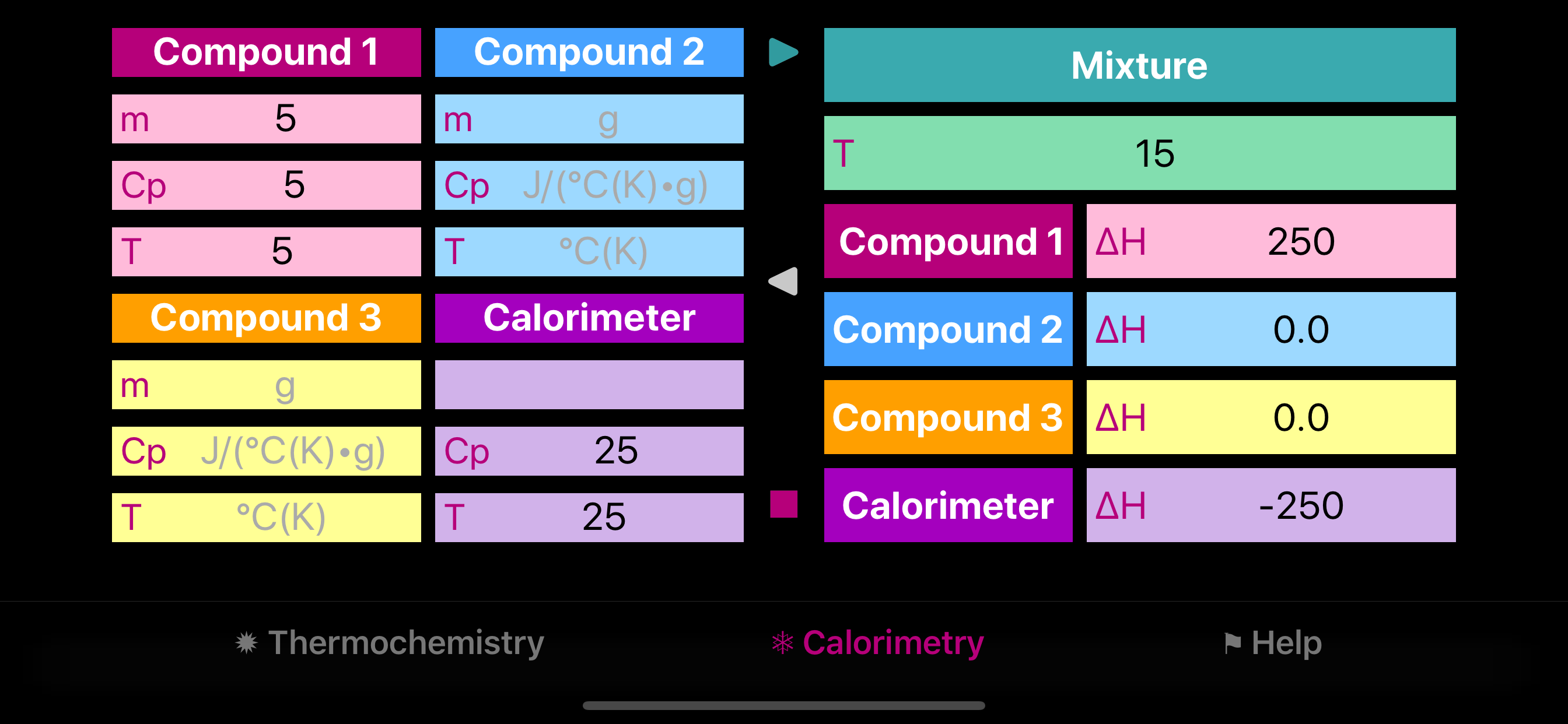
Calorimetry section provides means for evaluation of the heat capacity of calorimeter and for finding equilibrium temperature of mixed system. Forward arrow button sets the final temperature of the mixture. Backward arrow button sets missing temperature or heat capacity of one of the components. Enthalpy values show heat flow for each component.
Example of problems solved by application (screenshots):
Problem 1: Calculate the amount of energy required to change 100.0 g of ice at -15.0 °C to steam at 125.0 °C. Known values:
Heat of melting = 334.16 J g¯1
Heat of vaporization = 2259 J g¯1
specific heat capacity for solid water (ice) = 2.06 J g¯1 K¯1
specific heat capacity for liquid water = 4.184 J g¯1 K¯1
specific heat capacity for gaseous water (steam) = 2.02 J g¯1K¯1
Solution:
1) Heating of 100.0 g of ice from -15.0°C to 0.0°C:
(100.0 g) (15.0 K) (2.06 J g¯1 K¯1) = 3090 J
2) Melting of 100.0 g of ice:
(100.0 g) (334.16 J g¯1) = 33416 J
3) Heating of 100.0 g of liquid water from zero to 100.0 Celsius:
(100.0 g) (100.0 K) (4.184 J g¯1 K¯1) = 41840 J
4) Evaporations of 100.0 g of liquid:
(100.0 g) (2259 J g¯1) = 225900 J
5) Heating of 100.0 g of steam from 100.0 to 125.0 Celsius:
(100.0 g) (25.0 K) (2.02 J g¯1 K¯1) = 5050 J
6) Summation of the results:
3090 + 33416 + 41840 + 225900 + 5050 = 309.3 kJ
Problem 2: Determine the heat capacity of a coffee-cup calorimeter. During calibration 100.0 g of water at 58.5 °C has been added to 100.0 g of water, already in the calorimeter, at 22.8 °C. Calculate the heat capacity of the calorimeter in J/°C, if final temperature of the water is 39.7 °C. (Specific heat of water is 4.184 J/g °C.)
Solution:
1) Heat given up by warm water:
q = (100.0 g) (18.8 °C) (4.184 J/g °C) = 7865.92 J
2) Heat absorbed by water in the calorimeter:
q = (100.0 g) (16.9 °C) (4.184 J/g °C) = 7070.96 J
3) The difference was absorbed by the calorimeter:
7865.92 - 7070.96 = 794.96 J
4) Calorimeter constant:
794.96 J / 16.9 °C = 47.0 J/°C
Problem 3: Determine the final temperature when 10.0 g of aluminum at 130.0 °C mixes with 200.0 grams of water at 25.0 °C.
Please note the starting temperature of the metal is above the boiling point of water. In reality, the sample may vaporize a tiny amount of water, but we will assume it does not for the purposes of the calculation.
Solution:
1) The colder water will warm up and the warmer metal will cool down. The whole mixture will equilibrate up at the same temperature. The energy which "flowed" out of the warmer metal equals the energy which "flowed" into the colder water:
Qaluminum = Qwater
(10) (130 - x) (0.901) = (200.0 )(x - 25) (4.18)
117.13 - 0.901x = 83.6x - 2090
x = 26.12 °C.
Important! Water didn’t cross temperature of phase transition – vaporization; otherwise calculation would be more complex.
Calculation of reaction standard Gibbs free energy:
For the general reaction aA + bB -> cC + dD
ΔG°rxn = cΔGf°(C) + dΔGf°(D) - aΔGf°(A) - bΔGf°(B)
Example: Calculate the Gibbs free energy for the following reaction at 25 °C.
Cu (s) + H2O (g) -> CuO (s) + H2 (g)
ΔG°rxn = ΔGf°(CuO (s)) – ΔGf°(H2O (g)) = (–129.7 kJ/mol) – (–228.6 kJ/mol)
= 98.9 kJ/mol
ΔGf° = 0; for elements in their standard state by definition.
At equilibrium, ΔG = 0!
Important points
Application uses dot as a decimal separator.
Special attention should be paid for units’ consistency.
Ausblenden
Mehr anzeigen...
App provides enthalpy values for each step. Negative enthalpy points to exothermic process – heat release, while positive one to endothermic - heat absorption. °C and K are interchangeable.
Calorimetry section provides means for evaluation of the heat capacity of calorimeter and for finding equilibrium temperature of mixed system. Forward arrow button sets the final temperature of the mixture. Backward arrow button sets missing temperature or heat capacity of one of the components. Enthalpy values show heat flow for each component.
Example of problems solved by application (screenshots):
Problem 1: Calculate the amount of energy required to change 100.0 g of ice at -15.0 °C to steam at 125.0 °C. Known values:
Heat of melting = 334.16 J g¯1
Heat of vaporization = 2259 J g¯1
specific heat capacity for solid water (ice) = 2.06 J g¯1 K¯1
specific heat capacity for liquid water = 4.184 J g¯1 K¯1
specific heat capacity for gaseous water (steam) = 2.02 J g¯1K¯1
Solution:
1) Heating of 100.0 g of ice from -15.0°C to 0.0°C:
(100.0 g) (15.0 K) (2.06 J g¯1 K¯1) = 3090 J
2) Melting of 100.0 g of ice:
(100.0 g) (334.16 J g¯1) = 33416 J
3) Heating of 100.0 g of liquid water from zero to 100.0 Celsius:
(100.0 g) (100.0 K) (4.184 J g¯1 K¯1) = 41840 J
4) Evaporations of 100.0 g of liquid:
(100.0 g) (2259 J g¯1) = 225900 J
5) Heating of 100.0 g of steam from 100.0 to 125.0 Celsius:
(100.0 g) (25.0 K) (2.02 J g¯1 K¯1) = 5050 J
6) Summation of the results:
3090 + 33416 + 41840 + 225900 + 5050 = 309.3 kJ
Problem 2: Determine the heat capacity of a coffee-cup calorimeter. During calibration 100.0 g of water at 58.5 °C has been added to 100.0 g of water, already in the calorimeter, at 22.8 °C. Calculate the heat capacity of the calorimeter in J/°C, if final temperature of the water is 39.7 °C. (Specific heat of water is 4.184 J/g °C.)
Solution:
1) Heat given up by warm water:
q = (100.0 g) (18.8 °C) (4.184 J/g °C) = 7865.92 J
2) Heat absorbed by water in the calorimeter:
q = (100.0 g) (16.9 °C) (4.184 J/g °C) = 7070.96 J
3) The difference was absorbed by the calorimeter:
7865.92 - 7070.96 = 794.96 J
4) Calorimeter constant:
794.96 J / 16.9 °C = 47.0 J/°C
Problem 3: Determine the final temperature when 10.0 g of aluminum at 130.0 °C mixes with 200.0 grams of water at 25.0 °C.
Please note the starting temperature of the metal is above the boiling point of water. In reality, the sample may vaporize a tiny amount of water, but we will assume it does not for the purposes of the calculation.
Solution:
1) The colder water will warm up and the warmer metal will cool down. The whole mixture will equilibrate up at the same temperature. The energy which "flowed" out of the warmer metal equals the energy which "flowed" into the colder water:
Qaluminum = Qwater
(10) (130 - x) (0.901) = (200.0 )(x - 25) (4.18)
117.13 - 0.901x = 83.6x - 2090
x = 26.12 °C.
Important! Water didn’t cross temperature of phase transition – vaporization; otherwise calculation would be more complex.
Calculation of reaction standard Gibbs free energy:
For the general reaction aA + bB -> cC + dD
ΔG°rxn = cΔGf°(C) + dΔGf°(D) - aΔGf°(A) - bΔGf°(B)
Example: Calculate the Gibbs free energy for the following reaction at 25 °C.
Cu (s) + H2O (g) -> CuO (s) + H2 (g)
ΔG°rxn = ΔGf°(CuO (s)) – ΔGf°(H2O (g)) = (–129.7 kJ/mol) – (–228.6 kJ/mol)
= 98.9 kJ/mol
ΔGf° = 0; for elements in their standard state by definition.
At equilibrium, ΔG = 0!
Important points
Application uses dot as a decimal separator.
Special attention should be paid for units’ consistency.
Screenshots
Thermochemistry Häufige Fragen
-
Ist Thermochemistry kostenlos?
Ja, Thermochemistry ist komplett kostenlos und enthält keine In-App-Käufe oder Abonnements.
-
Ist Thermochemistry seriös?
Nicht genügend Bewertungen, um eine zuverlässige Einschätzung vorzunehmen. Die App benötigt mehr Nutzerfeedback.
Danke für die Stimme -
Wie viel kostet Thermochemistry?
Thermochemistry ist kostenlos.
-
Wie hoch ist der Umsatz von Thermochemistry?
Um geschätzte Einnahmen der Thermochemistry-App und weitere AppStore-Einblicke zu erhalten, können Sie sich bei der AppTail Mobile Analytics Platform anmelden.

Benutzerbewertung
Die App ist in Australien noch nicht bewertet.

Bewertungsverlauf
Thermochemistry Bewertungen
App hat noch keine Bewertungen
Store-Rankings

Ranking-Verlauf
App-Ranking-Verlauf noch nicht verfügbar

Kategorien-Rankings
App ist noch nicht gerankt
Thermochemistry Konkurrenten
| Name | Downloads (30d) | Monatlicher Umsatz | Rezensionen | Bewertungen | Letzte Veröffentlichung | |
|---|---|---|---|---|---|---|
|
PVnRT - Ideal Gas Calculator
|
View
|
View
|
0
|
|
vor 5 Jahren | |

Energy Future Magazine
|
View
|
View
|
0
|
|
vor 8 Jahren | |

Organic Chemistry Review
|
View
|
View
|
0
|
|
vor 8 Jahren | |
|
Maths Guru
Your personal maths trainer
|
View
|
View
|
0
|
|
vor 3 Monaten | |
|
MCAT Biochem
|
View
|
View
|
0
|
|
vor 1 Monat | |
|
EduRedox - Chemistry Reactions
Chemical Handbook & Equations
|
View
|
View
|
0
|
|
vor 3 Jahren | |
|
Molecular Formula Generator
|
View
|
View
|
0
|
|
vor 6 Jahren | |
|
Physics Toolkit Pro
|
View
|
View
|
0
|
|
vor 6 Jahren | |
|
Thermodynamics Calculator lite
Número de Eckert Fourier Lewis
|
View
|
View
|
0
|
|
vor 1 Jahr | |
|
StudyOrgo
|
View
|
View
|
0
|
|
vor 6 Jahren |
Thermochemistry Installationen
Letzte 30 TageThermochemistry Umsatz
Letzte 30 TageThermochemistry Einnahmen und Downloads
Gewinnen Sie wertvolle Einblicke in die Leistung von Thermochemistry mit unserer Analytik.
Melden Sie sich jetzt an, um Zugriff auf Downloads, Einnahmen und mehr zu erhalten.
Melden Sie sich jetzt an, um Zugriff auf Downloads, Einnahmen und mehr zu erhalten.
App-Informationen
- Kategorie
- Education
- Herausgeber
-
Roman Volinsky
- Sprachen
- English
- Letzte Veröffentlichung
- 4.1 (vor 2 Monaten )
- Veröffentlicht am
- Mar 24, 2016 (vor 8 Jahren )
- Auch verfügbar in
- Australien, Belgien, Kanada, Chile, Dänemark, Frankreich, Vereinigtes Königreich, Mexiko, Malaysia, Niederlande, Neuseeland, Saudi-Arabien, Schweden, Thailand, Vereinigte Staaten, Südafrika
- Zuletzt aktualisiert
- vor 6 Tagen
This page includes copyrighted content from third parties, shared solely for commentary and research in accordance with fair use under applicable copyright laws. All trademarks, including product, service, and company names or logos, remain the property of their respective owners. Their use here falls under nominative fair use as outlined by trademark laws and does not suggest any affiliation with or endorsement by the trademark holders.
- © 2025 AppTail.
- Unterstützung
- Privacy
- Terms
- All Apps